Pharmacology: Probing the pores in membrane vesicles
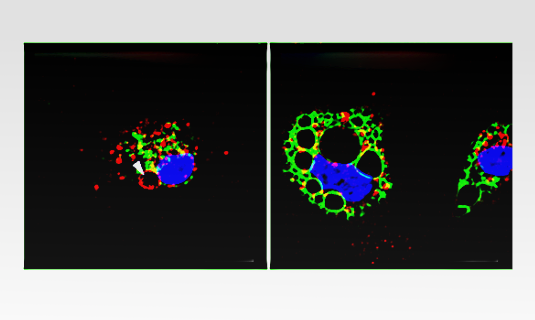 Microscopic image of mouse immune cells. Early endosomes are stained red, late endosomes and lysosomes green. The molecule YM201636 selectively enlarges late endosomes and lysosomes (right), while a combination of two biotoxins selectively enlarges only early endosomes (left). Image: C. Grimm, E. Butz
Microscopic image of mouse immune cells. Early endosomes are stained red, late endosomes and lysosomes green. The molecule YM201636 selectively enlarges late endosomes and lysosomes (right), while a combination of two biotoxins selectively enlarges only early endosomes (left). Image: C. Grimm, E. Butz
Tiny membrane-bound vesicles, known as endosomes and lysosomes, serve as vehicles for the transport of protein cargoes within animal cells. Embedded in the vesicle membranes are proteins called ion channels, which control the passage of electrically charged atomic particles (‘ions’) into and out of these intracellular organelles. Defects in these proteins play a central role in the pathogenesis of many diseases, and dissection of their molecular functions is vital for the development of effective therapies for these disorders. PD Christian Grimm and Professor Christian Wahl-Schott of the Department of Pharmacy (Director: Professor Martin Biel) at LMU Munich are among Europe’s leading specialists in the use of the so-called patch-clamp technique for the study of ion channels in cell membranes. In the latest issue of the journal Nature Protocols, they describe how they have adapted the method for use with endolysosomal vesicles. In a second study, published in the journal Cell Chemical Biology, they go on to demonstrate how patch clamping can be applied to specific functional classes of transport vesicles. This breakthrough opens up entirely new perspectives for the characterization of ion channels and the mechanisms that regulate them.
The endolysosomal system is made up of three distinct classes of endosomes – early, late and recycling endosomes – as well as lysosomes. These various types of vesicles have different functions. Early endosomal vesicles are formed by infolding of patches of the cell membrane in response to activation of e.g. receptor proteins by extracellular signaling molecules. This enables the receptors to be removed from the surface and detached from their activating ligands. The receptors are then either passed on to recycling endosomes and returned to the cell membrane, or delivered to late endosomes that convey them to lysosomes, where they are enzymatically degraded. This trafficking system participates in numerous metabolic processes and plays a vital role in the regulation of heavy-metal metabolism and in the correct localization of specific membrane receptors. The ion channels found in vesicular membranes are intimately involved in all of these operations. “According to studies of their protein populations, lysosomes and endosomes contain up to 70 distinct ion-channel transport proteins,” Grimm says.
The patch-clamp method allows one to measure the passage of charged particles through single ion channels in membrane patches, and hence to determine whether the channel is active or inactive. To do so, one draws a tiny patch of membrane into a micropipette by applying weak suction, thus ensuring that a tight seal is formed between the membrane and the wall of the pipette. With the aid of a microelectrode, one can then apply a test voltage and pass current through any ion channels present in the patch. “The problem is that, in their normal state, vesicles are too small to be accessed via a patch pipette, so they must be enlarged before any measurements can be carried out,” says Grimm. However, the pharmacological tools that have so far been used for this purpose act indiscriminately on all endolysosomal vesicle types. In an effort to discover more specific agents, the LMU researchers screened a library of compounds and identified a particular combination of two biological toxins that selectively induces the enlargement of early endosomes by causing them to fuse with one another. In addition, they were able to demonstrate that a third molecule selectively enlarges late endosomes and lysosomes. Notably, none of these substances acts on recycling endosomes.
“This represents a significant advance, because we now have two sets of tools that enable us to employ more specific approaches and ask which channels are active in which types of vesicle,” Grimm explains. Indeed, he and his colleagues have used the new agents to show that the so-called TRPML3 ion channel, which controls the passage of positively charged cations and the pH (level of acidity) within vesicles, is active in both early and late endosomes and lysosomes. In contrast, the related TRPML1 channel is found in late endosomes and lysosomes, but not in early endosomes. Mutations in TRPML channels contribute to the pathogenesis of severe congenital conditions such as mucolipidoses, a rare class of metabolic diseases that impairs nervous system function. “Thanks to our extension of patch clamping to intracellular vesicles, we can now address these ion channels with greater selectivity. This is also important in the search for substances that can specifically inhibit the functions of particular ion channels for therapeutic purposes,” Grimm points out.
Nature Protocols 2017
Cell Chemical Biology 2017