Immunology: A cautionary tale
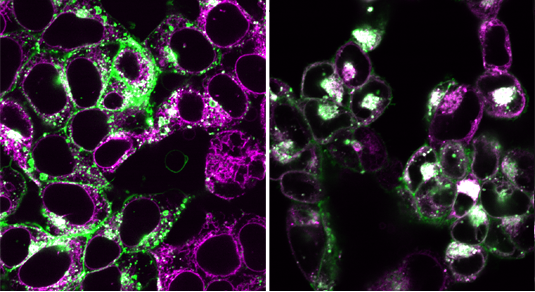 Human embryonic kidney cells. In the restimulated state (left) STING (green) is not located at the lysosome (magenta), therefore the colors do not overlap. After activation (right) STING migrates to the lysosomes and overlapping of magenta and green results in white. Image: V. Hornung
Human embryonic kidney cells. In the restimulated state (left) STING (green) is not located at the lysosome (magenta), therefore the colors do not overlap. After activation (right) STING migrates to the lysosomes and overlapping of magenta and green results in white. Image: V. Hornung
In eukaryotic cells, the hereditary material DNA is mostly confined to the nucleus whereas the presence of DNA outside of the nucleus in the cytoplasm is a sign of danger to the cell. Cytoplasmic DNA may originate from bacterial or viral sources and thus indicate an infection or it can be of endogenous origin and pinpoint to tissue injury. Consequently, its recognition by the innate immune system induces broadly inflammatory counter measures anddefense mechanisms. Researchers at LMU’s Gene Center, led by Professor Veit Hornung, have now elucidated the mechanisms that enable the innate immune system in human cells to recognize such misplaced DNA and trigger an inflammatory reaction. Disproving the common assumption that innate recognition processes are evolutionarily largely conserved, in this particular case, Hornung and colleagues reveal that human cells differ fundamentally from mouse cells with regards to cytosolic DNA recognition. The new findings appear in the latest issue of the leading journal Cell.
The presence of free DNA in the cytoplasm activates two distinct defense measures. The first is an antiviral immune response, mediated by induction of the synthesis and secretion of immunostimulatory messenger molecules called interferons. The second is a classical inflammatory reaction, which elicits symptoms such as fever, and localized swelling to allow recruitment of other immune cells to the site of emergency. A protein complex known as the inflammasome plays an important role in initiating the inflammation cascade, by activating the protein interleukin 1, a central mediator of inflammation. “We wanted to know how the inflammasome recognizes foreign DNA and triggers inflammation in human cells,” says Hornung. In previous studies he and his collaborators had shown that, in mouse cells, a particular DNA-binding receptor is required to activate the inflammasome.
“Our latest study shows that, in human myeloid cells, this receptor does not play a role in this process, because the functional module that recognizes foreign or misplaced DNA is hardwired in a different way, ”Hornung explains. In contrast to the mouse, the inflammasome of human myeloid cells is activated via the so-called cGAS-STING recognition mechanism, which is also responsible for triggering the innate immune response to the presence of viral DNA. The researchers have found now, that activation of the cGAS-STING pathway results in programmed cell death independently of the antiviral reaction. “When the activation of the cGAS-STING pathway exceeds a certain threshold, the STING protein induces the disintegration of a class of intracellular membrane vesicles called lysosomes”, Moritz Gaidt, a post-doc inHornung’s group and first author of the new study, explains.The resulting cell damage then activates the inflammasome to signal a local state of emergency by secreting interleukin 1. “By means of this inflammation reaction, the dying cell warns neighboring cells, which in turn recruit immune cells to the site of emergency,” says Hornung.
The findings not only shed new light on the function of the human immune system, they also underline the fact that experiments carried out in model organisms do not always reliably translate into human biology. “In an exemplary fashion our study demonstrates that it is worthwhile to study such signaling cascades directly in the human system,” says Hornung.
Cell 2017