Search
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
o-nitrophenolate (in DMF)  |
DMF | N Param.: 16.65 sN Param.: 0.70 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
o-nitrophenolate (in DMSO)  |
DMSO | N Param.: 15.48 sN Param.: 0.71 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
p-nitrophenolate (in DMF)  |
DMF | N Param.: 15.05 sN Param.: 0.80 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
p-nitrophenolate (in MeCN)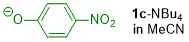  |
MeCN | N Param.: 15.14 sN Param.: 0.82 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
o-nitrophenolate (in MeCN)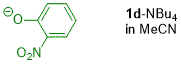  |
MeCN | N Param.: 15.83 sN Param.: 0.80 | J. Org. Chem. 2019, 84, 8837-8858 10.1021/acs.joc.9b01485 | |
cysteine (dianionic, in water)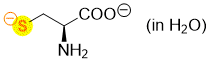  |
water | N Param.: 23.43 sN Param.: 0.42 | Org. Biomol. Chem. 2007, 5, 3814-3820 10.1039/b713778h | |
2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ)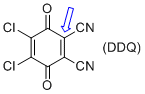  |
MeCN | E Param.: -3.66 | J. Am. Chem. Soc. 2013, 135, 12377-12387 10.1021/ja405890d | |
lithium (5-methylthiophen-2-yl)(4-(trifluoromethyl)phenyl)pinacolborate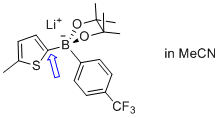  |
MeCN | N Param.: 6.24 sN Param.: 1.00 | Angew. Chem. Int. Ed. 2015, 54, 2780-2783 10.1002/anie.201410562 | |
potassium trifluoro(furan-2-yl)borate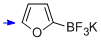  |
MeCN | N Param.: 5.99 sN Param.: 0.79 | J. Am. Chem. Soc. 2013, 135, 6317-6324 10.1021/ja4017655 | |
((2-(2-iodophenyl)propan-2-yl)oxy)(trifluoromethyl)sulfane  |
E Param.: -14.52 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
((2-phenylpropan-2-yl)oxy)(trifluoromethyl)sulfane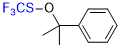  |
E Param.: -13.77 | Angew. Chem. Int. Ed. 2018, 57, 12690-12695 10.1002/anie.201805859 | ||
((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in CH2Cl2)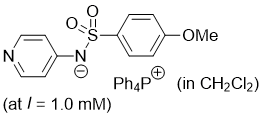  |
dichloromethane | N Param.: 19.63 sN Param.: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
((4-methoxyphenyl)sulfonyl)(pyridin-4-yl)amide (Ph4P+) (in MeCN)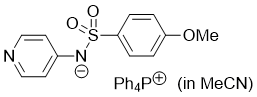  |
MeCN | N Param.: 17.28 sN Param.: 0.65 | J. Am. Chem. Soc. 2025, 147, 5043-5050 10.1021/jacs.4c14825 | |
((dimethylsilyl)methyl)trimethylsilane  |
dichloromethane | N Param.: 4.86 sN Param.: 0.64 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
((methylsilanediyl)bis(methylene))bis(trimethylsilane)  |
dichloromethane | N Param.: 4.91 sN Param.: 0.64 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
(1H-inden-1-yl)trimethylsilane (in CH2Cl2)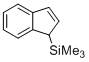  |
dichloromethane | N Param.: -0.10 sN Param.: 1.05 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
(1H-inden-1-yl)trimethylstannane (in CH2Cl2)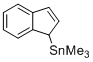  |
dichloromethane | N Param.: 6.68 sN Param.: 0.81 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
(1H-inden-1-yl)zinc(II) bromide*LiBr (in DMSO)  |
DMSO | N Param.: 15.60 sN Param.: 0.51 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
(1H-inden-1-yl)zinc(II) chloride*LiCl (in DMSO)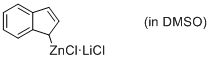  |
DMSO | N Param.: 18.10 sN Param.: 0.46 | Angew. Chem. Int. Ed. 2015, 54, 12497-12500 10.1002/anie.201501385 | |
(1S)-(-)-alpha-pinene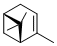  |
dichloromethane | N Param.: 0.68 sN Param.: 1.10 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
(2,2-diphenylethyl)diethyl(methyl)silane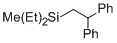  |
dichloromethane | N Param.: -6.40 sN Param.: 1.10 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
(2,3-dimethylbut-2-enyl)triethylsilane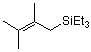  |
dichloromethane | N Param.: 3.15 sN Param.: 1.15 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
(2,4,6-trimethylphenyl)ethylium ion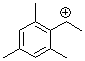  |
E Param.: 6.04 | Macromolecules 2005, 38, 33-40 10.1021/ma048389o | ||
(2-(4-methoxyphenyl)ethene-1,1-diyldisulfonyl)dibenzene  |
DMSO | E Param.: -13.88 | Chem. Asian J. 2012, 7, 1401-1407 10.1002/asia.201101046 | |
(2-([1,1'-biphenyl]-4-yl)ethyl)trimethylstannane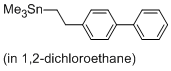  |
1,2-dichloroethane | N Param.: -0.80 sN Param.: 1.10 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
(2-methylallyl)benzene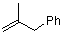  |
dichloromethane | N Param.: 0.25 sN Param.: 1.00 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
(2-methylallyl)dicarbonyl(cyclopentadienyl)iron(II)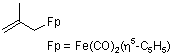  |
dichloromethane | N Param.: 8.45 sN Param.: 0.83 | Helv. Chim. Acta 2005, 88, 1754-1768 10.1002/hlca.200590137 | |
(2-methylallyl)tributylstannane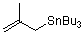  |
dichloromethane | N Param.: 7.48 sN Param.: 0.89 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
(2-methylallyl)trichlorosilane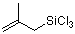  |
dichloromethane | N Param.: -0.57 sN Param.: 0.95 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
(2-methylallyl)trimethylsilane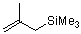  |
dichloromethane | N Param.: 4.41 sN Param.: 0.96 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
(2-methylallyl)triphenylgermane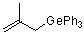  |
dichloromethane | N Param.: 4.67 sN Param.: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
(2-methylallyl)triphenylsilane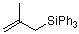  |
dichloromethane | N Param.: 4.17 sN Param.: 0.79 | J. Am. Chem. Soc. 2001, 123, 9500-9512 10.1021/ja010890y | |
(2-methylallyl)triphenylstannane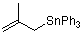  |
dichloromethane | N Param.: 5.13 sN Param.: 0.90 | Acc. Chem. Res. 2003, 36, 66-77 10.1021/ar020094c | |
(2-methylallyl)tris(trimethylsilyl)silane  |
dichloromethane | N Param.: 4.63 sN Param.: 0.87 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
(2-phenylethene-1,1-diyldisulfonyl)dibenzene  |
DMSO | E Param.: -12.93 | Chem. Asian J. 2012, 7, 1401-1407 10.1002/asia.201101046 | |
(2R,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one  |
MeCN | N Param.: 7.39 sN Param.: 1.00 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2S,5S)-5-benzyl-2-(tert-butyl)-3-methyl-1-((E)-styryl)imidazolidin-4-one  |
MeCN | N Param.: 5.80 sN Param.: 0.87 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 10.1002/anie.201201240 | |
(2S,5S)-5-benzyl-2-(tert-butyl)-3-methylimidazolidin-4-one  |
MeCN | N Param.: 5.44 sN Param.: 1.12 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2S,5S)-5-benzyl-3-methyl-2-(5-methylfuran-2-yl)imidazolidin-4-one  |
MeCN | N Param.: 8.76 sN Param.: 0.89 | J. Am. Chem. Soc. 2020, 142, 1526-1547 10.1021/jacs.9b11877 | |
(2S,5S)-5-benzyl-3-methyl-4-oxo-1-(3-phenylallylidene)-2-((E)-styryl)imidazolidin-1-ium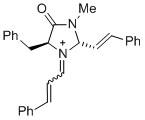  |
dichloromethane | E Param.: -5.90 | Asian J. Org. Chem. 2014, 3, 550-555 10.1002/ajoc.201402009 | |
(3,3,4,4,5,5,6,6,7,7,7-undecafluoro)-2-(trimethylsiloxy)hept-1-ene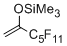  |
dichloromethane | N Param.: -3.52 sN Param.: 1.17 | Org. Lett. 2012, 14, 3990-3993 10.1021/ol301766w | |
(3-CF3)-tritylium ion  |
E Param.: 1.18 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | ||
(3-chlorobenzyl)dimethylsilane  |
dichloromethane | N Param.: 1.30 sN Param.: 0.75 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 | |
(3-Cl)-tritylium ion  |
E Param.: 1.06 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | ||
(3-Cl)3-tritylium ion  |
E Param.: 1.99 | J. Am. Chem. Soc. 2003, 125, 286-295 10.1021/ja021010y | ||
(3-F)-tritylium ion  |
E Param.: 1.01 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)2-tritylium ion  |
E Param.: 1.54 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)3-tritylium ion  |
E Param.: 2.07 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(3-F)4-tritylium ion  |
E Param.: 2.54 | Eur. J. Org. Chem. 2011, , 6470-6475 10.1002/ejoc.201100910 | ||
(4,4-dimethylpentyl)trimethylstannane  |
dichloromethane/MeCN mix | N Param.: -3.90 sN Param.: 1.10 | Chem. Eur. J. 2013, 19, 249-263 10.1002/chem.201202839 |
News
- 11/05/25:
Isochalcogenoureas (O, S, Se & Te derivatives) have been added (Angew. Chem. Int. Ed. 2025, EarlyView, e202514865). - 01/30/25:
Sesamol-derived ortho-quinone methides (o-QM) have been added (ChemEurJ 2024, e202403785 & OBC 2025, 23, 827-834) - 05/22/24:
Lactone enolates have been added (JOC 2024, 89, 6915-6928). - 11/13/24:
2-Methylene-1,2-dihydropyridines (2-pyNHOs) have been added (EurJOC 2024, 27, e202400373). - 05/21/24:
Mesoionic pyridinium-derived N-heterocyclic olefins (py-mNHOs) have been added (Angew. Chem. Int. Ed. 2024, 63, e202318283). - 10/19/23:
Enamines derived from 4-(alkylthio)-3-imidazolines and aldehydes have been added (ChemEurJ 2023, doi: 10.1002/chem.202302764). - 09/28/23:
Mesoionic N-heterocyclic olefins (mNHOs) have been added (Angew. Chem. Int. Ed. 2023, 62, e202309790). - 07/05/23:
S-Cyanomethylated imidazolidine-4-thione-derived enamines have been added (ChemComm 2023, 59, 8091). - 03/16/23:
Cyclic alpha-diazo carbonyl compounds added (EurJOC 2023, 26, e202300005) - 01/30/23:
NADH and NADPH reactivities (in water, pH 7) determined by Mayer and Moran have been added (OBC 2022, 21, 85-88). - 09/20/22:
Alkenyl boronate nucleophilicity by Liu, Ready and co-workers has been added (JACS 2022, 144, 16118). - 01/30/23:
Electrophilicities of diazo compounds in azo couplings (ChemEurJ 2022, 28, e202201376) - 01/30/23:
Diazocyclopentadiene added (Synthesis 2023, 55, 354-358) - 01/30/23:
Electrophilic indoles added (ChemCommun 2021, 57, 10071-10074) - 04/13/21:
Cyclic Michael acceptors added (Chem. Sci. 2021, 12, 4850-4865) - 05/21/21:
Thiophenolates (in DMSO) added (JOC 2021, 86, 5965-5972) - 05/06/20:
Heteroallenes added (JACS 2020, 142, 8383-8402). - 01/27/20:
Pyrrolidines and Imidazolidinones added (JACS 2020, 142, 1526-1547). - 05/06/20:
GSH reactivity added (Angew. Chem. Int. Ed. 2019, 58, 17704-17708) - 01/30/23:
Phenolates added (JOC 2019, 84, 8837-8858) - 04/24/18:
Ketones added (JACS 2018, 140, 5500). - 11/03/17:
Allyl-boron nucleophiles added (JACS 2017, 139, 15324) - 04/23/18:
Peroxide anions added (Angew. Chem. Int. Ed. 2017, 56, 13279). - 04/23/18:
Michael acceptors added (JACS 2017, 139, 13318). - 06/16/17:
Nucleophilicities of VNS reagents (JOC 2017, 82, 1011).
