

Organic Photochemistry and Energy ConversionPhotoreactions open exciting new reaction channels for the transformation of organic molecules. Many reactions that are impossible when only heat is used as a source of energy quickly proceed under irradiation. Thus, light can greatly simplify the synthesis of complex organic molecules, that can be biologically relevant, pharmaceutically active or of importance in the chemical industry. Also, organic photoreactions will likely play an important part in the production and conversion of renewable energy, be it as parts of organic photovoltaics or as sustainable sources of photo-generated fuels such as hydrogen or methanol. We study the light-absorbing molecules that are involved in these processes using highly correlated quantum chemical methods (CASSCF, CASPT2) and follow their dynamics after photoexcitation using semi-classical ab initio surface hopping or quantum dynamical methods. This allows us to discover which electronic states are involved in the photoreaction and how the excitation energy is transferred between these states. We then investigate how the properties of these states can be altered by substituent effects, solvent environments or ligands. This can aid in the design of experiments that lead to improved photocatalysts or the discovery of new photochemical reactions. Research Highlights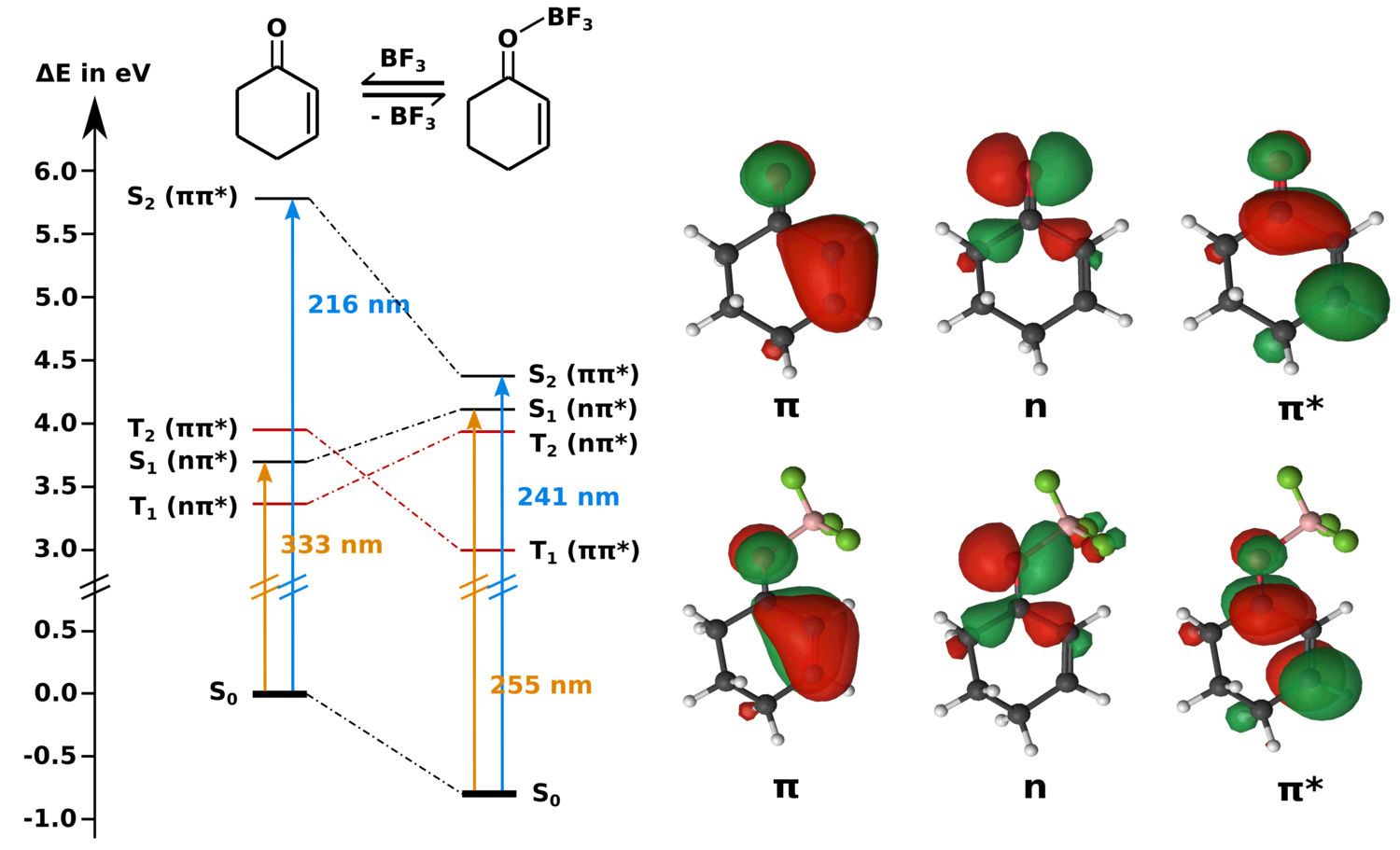
Activation of 2-Cyclohexenone by BF3 Coordination
Coordination of Lewis acids to the oxygen of enones stabilizes ππ* states and destabilizes nπ* states, which determines their photocatalytical activity. Angew. Chem. Int. Ed. 60 (2021), 10155-10163. Key Publications
|