Search
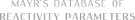
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
N-methyl-1-phenylmethanimine (in CH2Cl2)  |
dichloromethane | N Param.: 8.60 sN Param.: 0.77 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
1-phenyl-N-(phenylmethyl)methanimine (in CH2Cl2)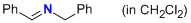  |
dichloromethane | N Param.: 7.90 sN Param.: 0.76 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
N-phenylpropan-2-imine (in CH2Cl2)  |
dichloromethane | N Param.: 9.53 sN Param.: 0.85 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
N-(phenylmethyl)propan-2-imine (in CH2Cl2)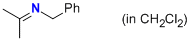  |
dichloromethane | N Param.: 11.13 sN Param.: 0.73 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
N-phenylcyclohexanimine (in CH2Cl2)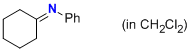  |
dichloromethane | N Param.: 8.80 sN Param.: 1.00 | Z. Naturforsch. B 2013, 68b, 693-699 10.5560/ZNB.2013-3085 | |
2-cinnamoyl-3-methyl-1-(perfluorophenyl)-1H-imidazol-3-ium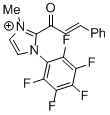  |
DMSO | E Param.: -10.09 | Top. Catal. 2018, 61, 585-590 10.1007/s11244-018-0[...] | |
1-(tert-butyl)-2-cinnamoyl-3-methyl-1H-imidazol-3-ium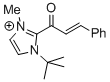  |
DMSO | E Param.: -11.80 | Top. Catal. 2018, 61, 585-590 10.1007/s11244-018-0[...] | |
2-cinnamoyl-1-mesityl-3-methyl-1H-imidazol-3-ium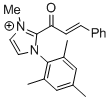  |
DMSO | E Param.: -11.48 | Top. Catal. 2018, 61, 585-590 10.1007/s11244-018-0[...] | |
2-cinnamoyl-1-(4-methoxyphenyl)-3-methyl-1H-imidazol-3-ium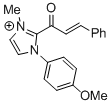  |
DMSO | E Param.: -11.79 | Top. Catal. 2018, 61, 585-590 10.1007/s11244-018-0[...] | |
2-cinnamoyl-1-(2,6-dimethoxyphenyl)-3-methyl-1H-imidazol-3-ium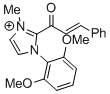  |
DMSO | E Param.: -12.02 | Top. Catal. 2018, 61, 585-590 10.1007/s11244-018-0[...] | |
1-((diphenylmethylene)amino)-2-ethoxy-2-oxoethan-1-ide (in DMSO)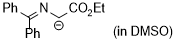  |
DMSO | N Param.: 26.95 sN Param.: 0.52 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
2-(tert-butoxy)-1-((diphenylmethylene)amino)-2-oxoethan-1-ide (in DMSO)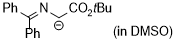  |
DMSO | N Param.: 27.77 sN Param.: 0.47 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
1-(benzylideneamino)-2-ethoxy-2-oxoethan-1-ide (in DMSO)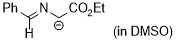  |
DMSO | N Param.: 29.10 sN Param.: 0.50 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
1-((4-chlorobenzylidene)amino)-2-ethoxy-2-oxoethan-1-ide (in DMSO)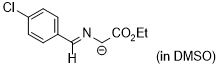  |
DMSO | N Param.: 29.02 sN Param.: 0.49 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
2-(benzylideneamino)-1-ethoxy-1-oxopropan-2-ide (in DMSO)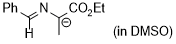  |
DMSO | N Param.: 30.82 sN Param.: 0.41 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
cyano((diphenylmethylene)amino)methanide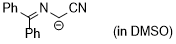  |
DMSO | N Param.: 29.50 sN Param.: 0.50 | Tetrahedron 2019, 75, 459-463 10.1016/j.tet.2018.1[...] | |
(Z)-1,2-diphenyl-1-(trimethylsiloxy)ethene (in MeCN)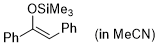  |
MeCN | N Param.: 3.00 sN Param.: 0.83 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
(Z)-1-phenyl-1-(trimethylsiloxy)propene (in MeCN)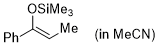  |
MeCN | N Param.: 5.18 sN Param.: 0.94 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
3-(trimethylsiloxy)-1H-indene (in MeCN)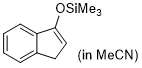  |
MeCN | N Param.: 7.32 sN Param.: 0.82 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
1-(trimethylsiloxy)-3,4-dihydronaphthalene (in MeCN)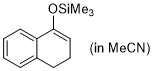  |
MeCN | N Param.: 5.06 sN Param.: 0.91 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
3-pyrrolidino-1H-indene (in MeCN)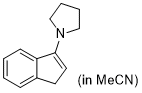  |
MeCN | N Param.: 15.27 sN Param.: 0.93 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
1-pyrrolidino-3,4-dihydronaphthalene (in MeCN)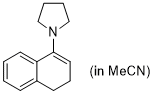  |
MeCN | N Param.: 14.09 sN Param.: 0.66 | Synthesis 2019, 51, 1157-1170 10.1055/s-0037-1611634 | |
diazocyclopentadiene (in CH2Cl2)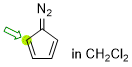  |
dichloromethane | N Param.: 4.84 sN Param.: 1.06 | Synthesis 2023, 55, 354-358 10.1055/s-0041-1737327 | |
pyridine (in MeCN)  |
MeCN | N Param.: 13.60 sN Param.: 0.60 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2-methylpyridine (in CH2Cl2)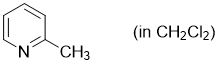  |
dichloromethane | N Param.: 10.52 sN Param.: 0.78 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2-methylpyridine (in MeCN)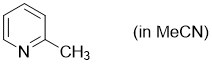  |
MeCN | N Param.: 10.98 sN Param.: 0.66 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,6-dimethylpyridine (in CH2Cl2)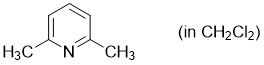  |
dichloromethane | N Param.: 9.87 sN Param.: 0.68 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,6-dimethylpyridine (in MeCN)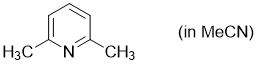  |
MeCN | N Param.: 9.11 sN Param.: 0.69 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,4,6-trimethylpyridine (in CH2Cl2)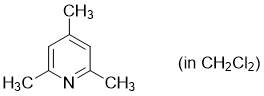  |
dichloromethane | N Param.: 9.34 sN Param.: 0.71 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
2,4,6-trimethylpyridine (in MeCN)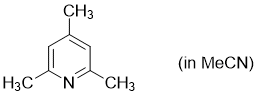  |
MeCN | N Param.: 9.39 sN Param.: 0.60 | Synthesis 2017, 49, 3495-3504 10.1055/s-0036-1590504 | |
Me2C=C=N(+)Me2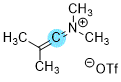  |
E Param.: -2.80 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
Ph2C=C=N(+)Me2  |
E Param.: -1.90 | Synthesis 2025, 57, 3251-3262 10.1055/a-2649-1999 | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = Ph)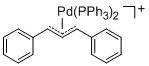  |
E Param.: -14.14 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 3,5-difluorophenyl)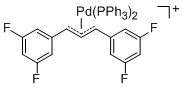  |
E Param.: -14.21 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 | ||
[(1,3-Diarylallyl)Pd(PPh3)2]+ (Aryl = 4-(dimethylamino)phenyl)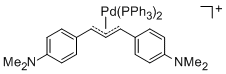  |
E Param.: -14.46 | Organometallics 2012, 31, 2416-2424 10.1021/om3000357 | ||
2,3,4,5,6,6-hexachlorocyclohexa-2,4-dien-1-one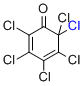  |
MeCN | E Param.: -6.75 | Org. Lett. 2010, 12, 2238-2241 10.1021/ol100592j | |
5,7,7-trichloro-8(7H)-quinolinone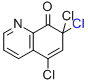  |
MeCN | E Param.: -10.48 | Org. Lett. 2010, 12, 2238-2241 10.1021/ol100592j | |
2,2,4-trichloro-1(2H)-naphthalenone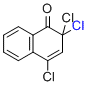  |
MeCN | E Param.: -11.24 | Org. Lett. 2010, 12, 2238-2241 10.1021/ol100592j | |
allyltris(trimethylsilyl)silane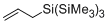  |
dichloromethane | N Param.: 1.95 sN Param.: 0.98 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
(2-methylallyl)tris(trimethylsilyl)silane  |
dichloromethane | N Param.: 4.63 sN Param.: 0.87 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
2-(tris(trimethylsilyl)siloxy)propene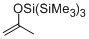  |
dichloromethane | N Param.: 6.04 sN Param.: 0.82 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
1-(tris(trimethylsilyl)siloxy)cyclohexene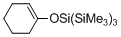  |
dichloromethane | N Param.: 5.07 sN Param.: 0.91 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
(tris(trimethylsilyl)siloxy)ethene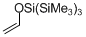  |
dichloromethane | N Param.: 4.01 sN Param.: 0.83 | Org. Lett. 2010, 12, 5206-5209 10.1021/ol102220e | |
2-Me super-dmap (in MeCN)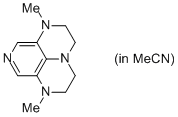  |
MeCN | N Param.: 16.65 sN Param.: 0.58 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Et super-dmap (in MeCN)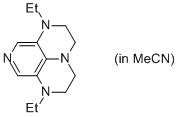  |
MeCN | N Param.: 16.81 sN Param.: 0.60 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Bn super-dmap (in MeCN)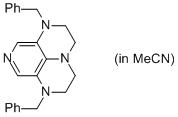  |
MeCN | N Param.: 17.69 sN Param.: 0.57 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Ac super-dmap (in MeCN)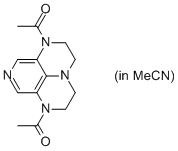  |
MeCN | N Param.: 15.39 sN Param.: 0.60 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
2-Bz super-dmap (in MeCN)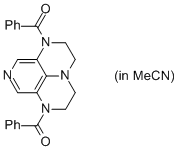  |
MeCN | N Param.: 14.19 sN Param.: 0.67 | Org. Lett. 2011, 13, 530-533 10.1021/ol1029589 | |
dipp Imd boronate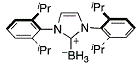  |
dichloromethane | N Param.: 9.55 sN Param.: 0.81 | Org. Lett. 2012, 14, 82-85 10.1021/ol202836p | |
Me2 Imd boronate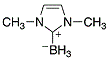  |
dichloromethane | N Param.: 11.88 sN Param.: 0.71 | Org. Lett. 2012, 14, 82-85 10.1021/ol202836p |
News
- 11/05/25:
Isochalcogenoureas (O, S, Se & Te derivatives) have been added (Angew. Chem. Int. Ed. 2025, EarlyView, e202514865). - 01/30/25:
Sesamol-derived ortho-quinone methides (o-QM) have been added (ChemEurJ 2024, e202403785 & OBC 2025, 23, 827-834) - 05/22/24:
Lactone enolates have been added (JOC 2024, 89, 6915-6928). - 11/13/24:
2-Methylene-1,2-dihydropyridines (2-pyNHOs) have been added (EurJOC 2024, 27, e202400373). - 05/21/24:
Mesoionic pyridinium-derived N-heterocyclic olefins (py-mNHOs) have been added (Angew. Chem. Int. Ed. 2024, 63, e202318283). - 10/19/23:
Enamines derived from 4-(alkylthio)-3-imidazolines and aldehydes have been added (ChemEurJ 2023, doi: 10.1002/chem.202302764). - 09/28/23:
Mesoionic N-heterocyclic olefins (mNHOs) have been added (Angew. Chem. Int. Ed. 2023, 62, e202309790). - 07/05/23:
S-Cyanomethylated imidazolidine-4-thione-derived enamines have been added (ChemComm 2023, 59, 8091). - 03/16/23:
Cyclic alpha-diazo carbonyl compounds added (EurJOC 2023, 26, e202300005) - 01/30/23:
NADH and NADPH reactivities (in water, pH 7) determined by Mayer and Moran have been added (OBC 2022, 21, 85-88). - 09/20/22:
Alkenyl boronate nucleophilicity by Liu, Ready and co-workers has been added (JACS 2022, 144, 16118). - 01/30/23:
Electrophilicities of diazo compounds in azo couplings (ChemEurJ 2022, 28, e202201376) - 01/30/23:
Diazocyclopentadiene added (Synthesis 2023, 55, 354-358) - 01/30/23:
Electrophilic indoles added (ChemCommun 2021, 57, 10071-10074) - 04/13/21:
Cyclic Michael acceptors added (Chem. Sci. 2021, 12, 4850-4865) - 05/21/21:
Thiophenolates (in DMSO) added (JOC 2021, 86, 5965-5972) - 05/06/20:
Heteroallenes added (JACS 2020, 142, 8383-8402). - 01/27/20:
Pyrrolidines and Imidazolidinones added (JACS 2020, 142, 1526-1547). - 05/06/20:
GSH reactivity added (Angew. Chem. Int. Ed. 2019, 58, 17704-17708) - 01/30/23:
Phenolates added (JOC 2019, 84, 8837-8858) - 04/24/18:
Ketones added (JACS 2018, 140, 5500). - 11/03/17:
Allyl-boron nucleophiles added (JACS 2017, 139, 15324) - 04/23/18:
Peroxide anions added (Angew. Chem. Int. Ed. 2017, 56, 13279). - 04/23/18:
Michael acceptors added (JACS 2017, 139, 13318). - 06/16/17:
Nucleophilicities of VNS reagents (JOC 2017, 82, 1011).
