Search
| Name | Solvent |
Reactivity Parameters |
Classification |
Reference (title or year) |
|---|---|---|---|---|
beta-piperidinium-peroxypropionate  |
water | N Param.: 13.94 sN Param.: 0.62 | Eur. J. Org. Chem. 2018, , 6010-6017 10.1002/ejoc.201801158 | |
peroxy-beta-alanate  |
water | N Param.: 14.07 sN Param.: 0.56 | Eur. J. Org. Chem. 2018, , 6010-6017 10.1002/ejoc.201801158 | |
peroxy-GABA  |
water | N Param.: 14.33 sN Param.: 0.57 | Eur. J. Org. Chem. 2018, , 6010-6017 10.1002/ejoc.201801158 | |
2,6-di-tert-butyl-4-(2,2,2-trifluoroethylidene)cyclohexa-2,5-dien-1-one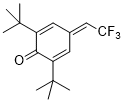  |
DMSO | E Param.: -11.68 | Eur. J. Org. Chem. 2020, , 3812-3817 10.1002/ejoc.202000295 | |
3-diazoindolin-2-one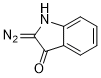  |
dichloromethane | N Param.: 3.16 sN Param.: 1.03 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindan-1-one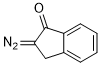  |
dichloromethane | N Param.: 5.61 sN Param.: 0.65 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazocyclohexanone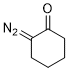  |
dichloromethane | N Param.: 3.44 sN Param.: 0.83 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-tetralone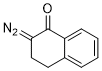  |
dichloromethane | N Param.: 3.51 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazoindandione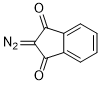  |
dichloromethane | N Param.: 0.16 sN Param.: 0.86 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazo-1-benzosuberone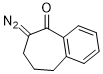  |
dichloromethane | N Param.: 2.72 sN Param.: 0.96 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
2-diazobenzothiophen-3(2H)-one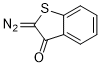  |
dichloromethane | N Param.: 0.40 sN Param.: 0.93 | Eur. J. Org. Chem. 2023, 26, e202300005 10.1002/ejoc.202300005 | |
Umemoto I (triflate)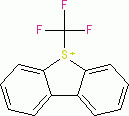  |
E Param.: -13.08 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto I (tetrafluoroborate)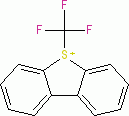  |
E Param.: -13.39 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
Umemoto II (triflate)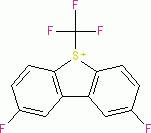  |
E Param.: -12.80 | Eur. J. Org. Chem. 2024, 27, e202400085 10.1002/ejoc.202400085 | ||
1,3,5-trimethyl-2-methylene-1,2-dihydropyridine (in DMSO)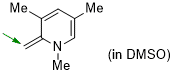  |
DMSO | N Param.: 21.16 sN Param.: 0.59 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1,3-dimethyl-2-methylene-1,2-dihydropyridine (in DMSO)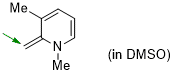  |
DMSO | N Param.: 19.91 sN Param.: 0.62 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1,3-dimethyl-2-methylene-1,2-dihydropyridine (in MeCN)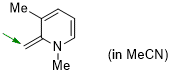  |
MeCN | N Param.: 19.69 sN Param.: 0.61 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1,6-dimethyl-2-methylene-1,2-dihydropyridine (in DMSO)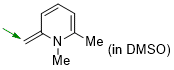  |
DMSO | N Param.: 20.76 sN Param.: 0.60 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1-methyl-2-methylene-3-phenyl-1,2-dihydropyridine (in DMSO)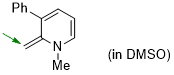  |
DMSO | N Param.: 19.41 sN Param.: 0.61 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 | |
1-(4-(tert-butyl)phenyl)-2-methylene-4,6-diphenyl-1,2-dihydropyridine (in DMSO)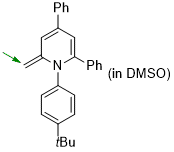  |
DMSO | N Param.: 19.36 sN Param.: 0.68 | Eur. J. Org. Chem. 2024, 27, e202400373 10.1002/ejoc.202400373 |
News
- 11/05/25:
Isochalcogenoureas (O, S, Se & Te derivatives) have been added (Angew. Chem. Int. Ed. 2025, EarlyView, e202514865). - 01/30/25:
Sesamol-derived ortho-quinone methides (o-QM) have been added (ChemEurJ 2024, e202403785 & OBC 2025, 23, 827-834) - 05/22/24:
Lactone enolates have been added (JOC 2024, 89, 6915-6928). - 11/13/24:
2-Methylene-1,2-dihydropyridines (2-pyNHOs) have been added (EurJOC 2024, 27, e202400373). - 05/21/24:
Mesoionic pyridinium-derived N-heterocyclic olefins (py-mNHOs) have been added (Angew. Chem. Int. Ed. 2024, 63, e202318283). - 10/19/23:
Enamines derived from 4-(alkylthio)-3-imidazolines and aldehydes have been added (ChemEurJ 2023, doi: 10.1002/chem.202302764). - 09/28/23:
Mesoionic N-heterocyclic olefins (mNHOs) have been added (Angew. Chem. Int. Ed. 2023, 62, e202309790). - 07/05/23:
S-Cyanomethylated imidazolidine-4-thione-derived enamines have been added (ChemComm 2023, 59, 8091). - 03/16/23:
Cyclic alpha-diazo carbonyl compounds added (EurJOC 2023, 26, e202300005) - 01/30/23:
NADH and NADPH reactivities (in water, pH 7) determined by Mayer and Moran have been added (OBC 2022, 21, 85-88). - 09/20/22:
Alkenyl boronate nucleophilicity by Liu, Ready and co-workers has been added (JACS 2022, 144, 16118). - 01/30/23:
Electrophilicities of diazo compounds in azo couplings (ChemEurJ 2022, 28, e202201376) - 01/30/23:
Diazocyclopentadiene added (Synthesis 2023, 55, 354-358) - 01/30/23:
Electrophilic indoles added (ChemCommun 2021, 57, 10071-10074) - 04/13/21:
Cyclic Michael acceptors added (Chem. Sci. 2021, 12, 4850-4865) - 05/21/21:
Thiophenolates (in DMSO) added (JOC 2021, 86, 5965-5972) - 05/06/20:
Heteroallenes added (JACS 2020, 142, 8383-8402). - 01/27/20:
Pyrrolidines and Imidazolidinones added (JACS 2020, 142, 1526-1547). - 05/06/20:
GSH reactivity added (Angew. Chem. Int. Ed. 2019, 58, 17704-17708) - 01/30/23:
Phenolates added (JOC 2019, 84, 8837-8858) - 04/24/18:
Ketones added (JACS 2018, 140, 5500). - 11/03/17:
Allyl-boron nucleophiles added (JACS 2017, 139, 15324) - 04/23/18:
Peroxide anions added (Angew. Chem. Int. Ed. 2017, 56, 13279). - 04/23/18:
Michael acceptors added (JACS 2017, 139, 13318). - 06/16/17:
Nucleophilicities of VNS reagents (JOC 2017, 82, 1011).
