Prof. Dr. Rudolf Knorr
Department Chemie
Ludwig-Maximilians-Universität München
Prof. Dr. Rudolf Knorr
Butenandtstr. 5-13 (Haus F)
D-81377 München-Grosshadern
Germany
Telefon: +49 89 2180 77696
Veröffentlichungen
-
Alkylidenecarbenes, Alkylidenecarbenoids, and Competing Species:
Which Is Responsible for Vinylic Nucleophilic Substitution, [1 + 2] Cycloadditions,
1,5-CH Insertions, and the Fritsch-Buttenberg-Wiechell Rearrangement?
R. Knorr, Chem. Rev. 2004, 104, 3795-3849.
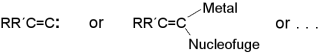
-
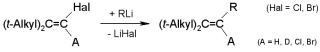
Carbenoid Chain Reactions: Substitutions by Organolithium Compounds at
Unactivated 1-Chloro-1-alkenes, R.Knorr, C. Pires, C. Behringer,
Th. Menke, J. Freudenreich, E. C. Rossmann, P. Böhrer,
J. Am. Chem. Soc. 2006, 128, 14845-14853.
-
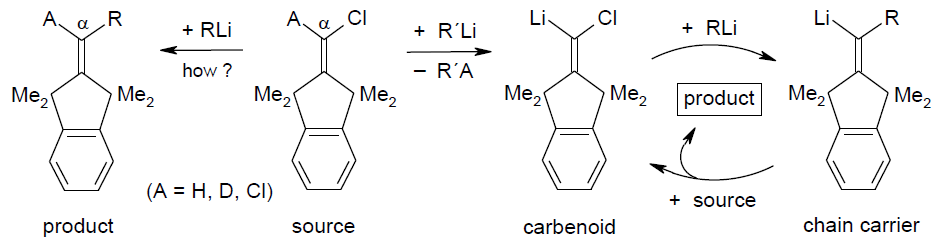
Carbenoid Chain Reactions through Proton, Deuteron, or Bromine
Transfer from Unactivated 1-Bromo-1-alkenes to Organolithium Compounds,
R. Knorr, C. Pires, J. Freudenreich, J. Org. Chem. 2007,
72, 6084-6090.
-
Unpaired Spin Densities from NMR Shifts and Magnetic Anisotropies of
Pseudotetrahedral Cobalt(II) and Nickel(II) Vinamidine Bis(chelates),
R. Knorr, H. Hauer, A. Weiss, H. Polzer, F. Ruf, P. Löw, P. Dvortsák,
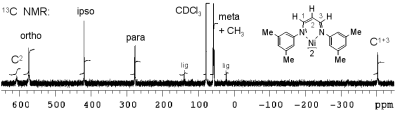
and P. Böhrer, Inorg. Chem. 2007, 46, 8379-8390.
-
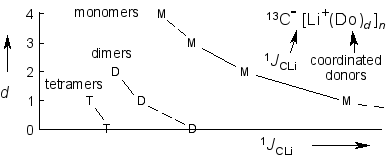
Microsolvation and 13C-Li NMR Coupling,
R. Knorr, Th. Menke, K. Ferchland, J. Mehlstäubl, D. S. Stephenson, J. Am. Chem. Soc. 2008, 130, 14179-14188
-
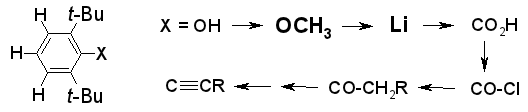
Easier Preparation of 2,6-Di-tert-butylphenyl Derivatives,
Rudolf Knorr, Eva Christine Rossmann, Monika Knittl,
Synthesis, 2010, 2124-2128.
-
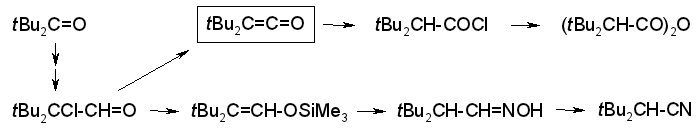
Shorter and Easier Syntheses of Di-tert-butylketene and Related gem-Di-tert-butyl Compounds,
Rudolf Knorr, Karsten-Olaf Hennig, Bernhard Schubert, and Petra Böhrer,
Eur. J. Org. Chem., 2010, 6651-6664.
-
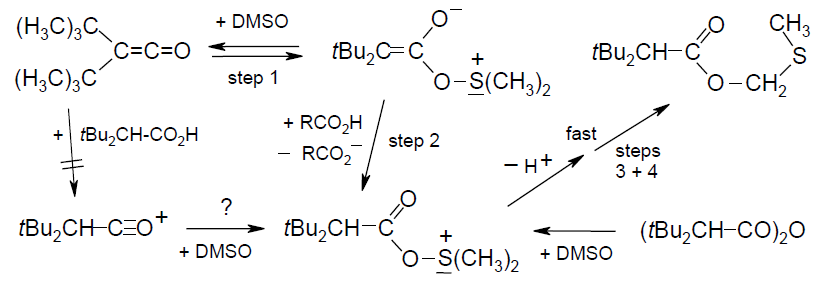
Acylation Mechanisms of DMSO/[D6]DMSO with Di-tert-butylketene and Its Congeners, Rudolf Knorr, Eur. J. Org. Chem. 2011, 6335-6342.
-
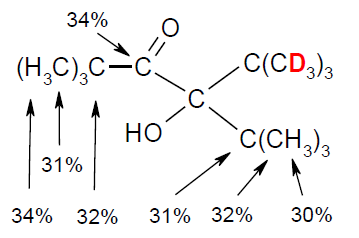
Deuterium Quantification Through Deuterium-Induced Remote 1H and 13C NMR Shifts, Rudolf Knorr and David S. Stephenson, Chem. Eur. J. 2012, 18, 7501-7505.
-
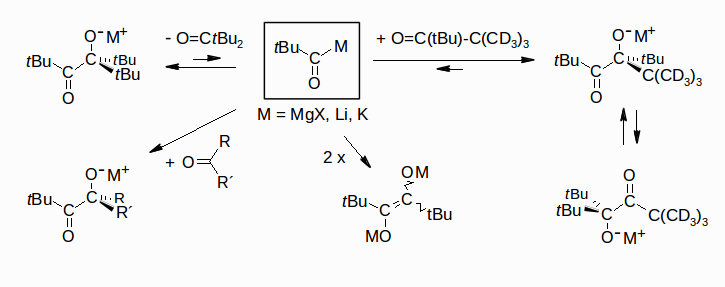
Pivaloylmetals (tBu-COM: M = Li, MgX, K) as Equilibrium Components, Rudolf Knorr, Gerald Böhrer, Bernhard Schubert, and Petra Böhrer, Chem. Eur. J. 2012, 18, 7506-7515.
-
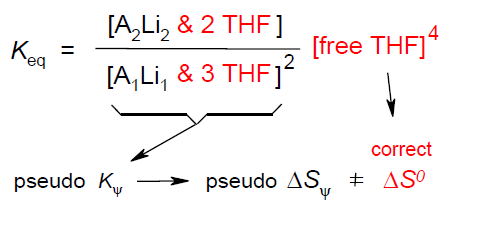
Entropies of Organolithium Aggregation based upon Measured Microsolvation Numbers; Rudolf Knorr, Thomas Menke, and Kathrin Ferchland, Organometallics 2013, 32, 468-472.
-
Pseudomonomolecular, Ionic sp2-Stereoinversion Mechanism of 1-Aryl-1-alkenyllithiums; Rudolf Knorr, Thomas Menke, Claudia Behringer, Kathrin Ferchland, Johann Mehlstäubl, and Ernst Lattke, Organometallics 2013, 32, 4070-4081.
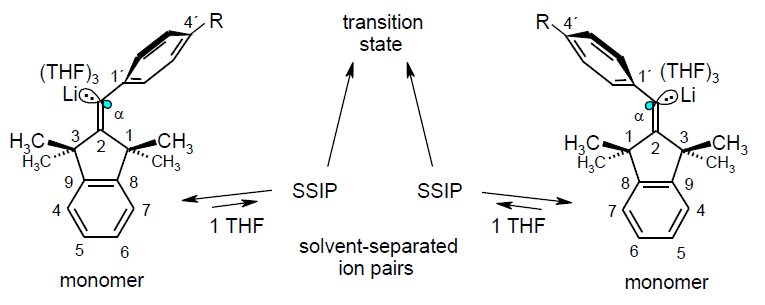
-
Carbenoid-mediated nucleophilic “hydrolysis” of 2-(dichloromethylidene)-1,1,3,3-tetramethylindane with DMSO participation, affording access to one-sidedly overcrowded ketone and bromoalkene descendants; Rudolf Knorr, Thomas Menke, Johannes Freudenreich, and Claudio Pires, Beilstein J. Org. Chem. 2014, 10, 307-315.
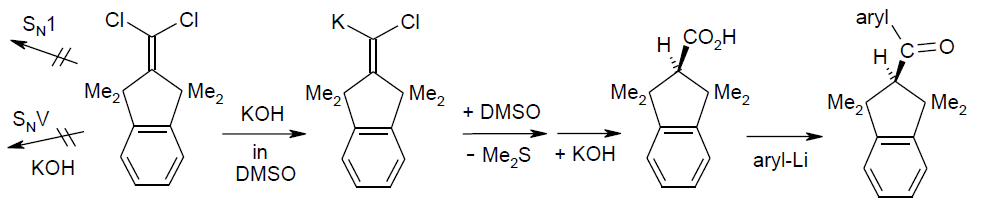
-
Ring expansion and vinylic nucleophilic substitution competing for (tert-alkyl)2C=C(Li)-Cl in carbenoid chain processes; Rudolf Knorr, Thomas Menke, Karsten-Olaf Hennig, Johannes Freudenreich, Petra Böhrer, and Bernhard Schubert, Tetrahedron 2014. 70, 2703-2710.
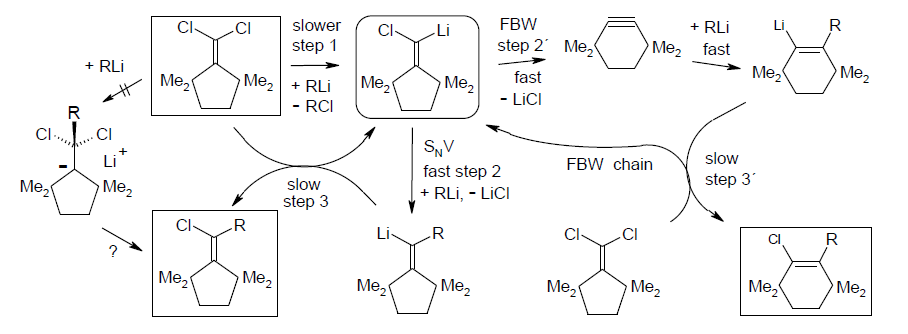
-
Highly syn selective addition of aqueous HBr to hydrophobically shielded arylalkynes; Rudolf Knorr, Eva C. Rossmann, Monika Knittl, and Petra Böhrer, Tetrahedron 2014. 70, 5332-5338.
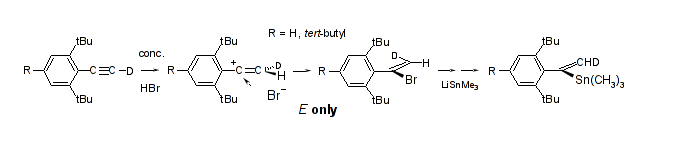
-
Microsolvation, aggregation, and pseudomonomolecular, ionic sp2-stereoinversion mechanism of two exocyclic β,β-di-tert-alkyl-α-arylvinyllithiums; Rudolf Knorr, Karsten-Olaf Hennig, Petra Böhrer, and Bernhard Schubert, J. Organomet. Chem. 2014, 767, 125-135.
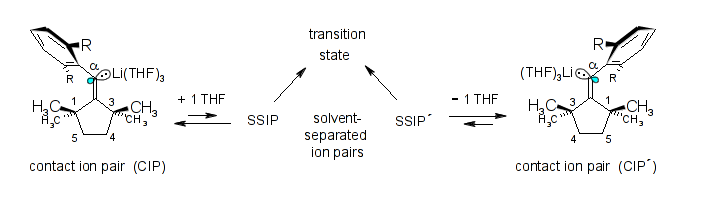
-
Microsolvation and sp2-stereoinversion of monomeric α-(2,6-di-tert-butylphenyl)vinyl-lithium as measured by NMR; R. Knorr, M. Knittl and E. C. Rossmann, Beilstein J. Org. Chem. 2014, 10, 2521-2530.
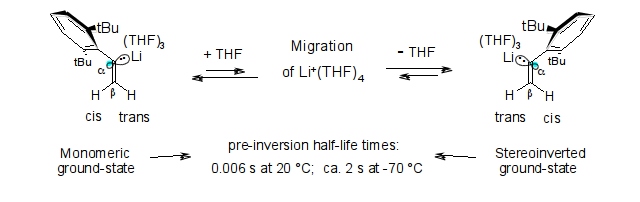
-
Microsolvation, Dimerization, and sp2-Stereoinversion of Monomeric α-(2,6-Diisopropylphenyl)vinyllithium; R. Knorr, J. Ruhdorfer, and P. Böhrer, Organometallics 2015, 34, 1038-1045.
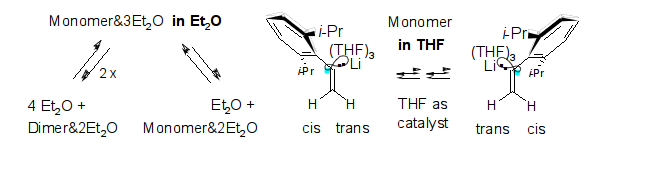
-
How Microsolvation Numbers at Li Control Aggregation Modes, sp2-Stereoinversion, and NMR Coupling Constants 2JH,H of H2C=C in α-(2,6-Dimethylphenyl)vinyllithium; R. Knorr, C. Behringer, E. Lattke, U. von Roman, and M. Knittl, J. Org. Chem. 2015, 60, 6313-6322.
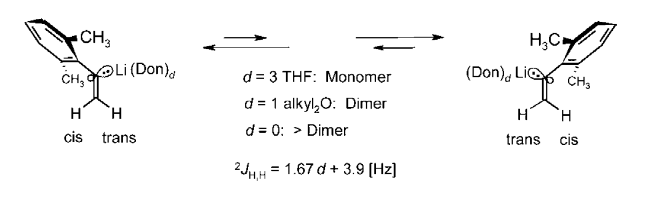
-
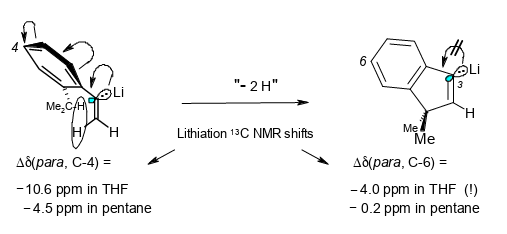
What can 13C and 1H NMR lithiation shifts tell us about the charge distribution in α-arylvinyllithium compounds?
Rudolf Knorr, Ernst Lattke, Jakob Ruhdorfer, Ulrich von Roman, Joachim Firl, and Petra Böhrer, J. Organomet. Chem. 2016, 824, 61–72.
-
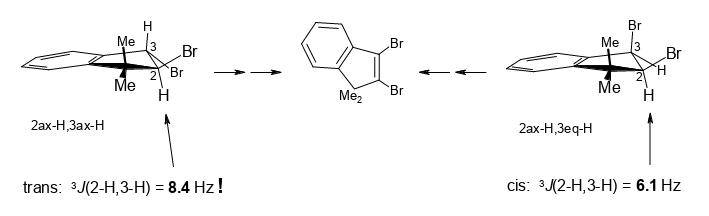
Unusual traits of cis and trans-2,3-dibromo-1,1-dimethylindane on the way from 1,1-dimethylindene to 2-bromo-, 3-bromo-, and 2,3-dibromo-1,1-dimethylindene.
Rudolf Knorr, David S. Stephenson, Ernst Lattke, Petra Böhrer and Jakob Ruhdorfer, Beilstein J. Org. Chem. 2016, 12, 1178–1184.
-
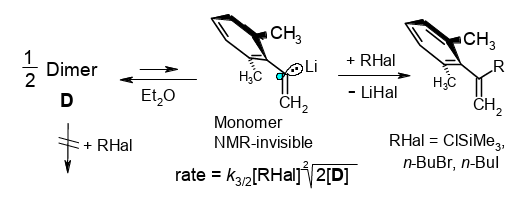
Kinetics of α-(2,6-Dimethylphenl)vinyllithium: How To Control Errors Caused by Inefficient Mixing with Pairs of Rapidly Competing Ketones.
Rudolf Knorr, Monika Knittl, Claudia Behringer, Jakob Ruhdorfer, and Petra Böhrer, J. Org. Chem. 2017, 82, 2843−2854.
-
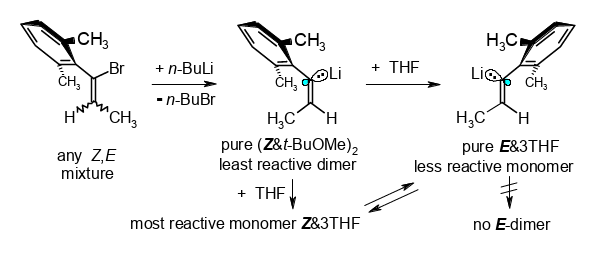
Doubly Diastereoconvergent Preparation and Microsolvation-Controlled Properties of (Z)- and (E)-1′-Lithio-1′-(2,6-dimethylphenyl)propenes.
Rudolf Knorr, Claudia Behringer, Monika Knittl, Ulrich von Roman, and Ernst Lattke, J. Am. Chem. Soc. 2017, 139, 4690−4703.
-
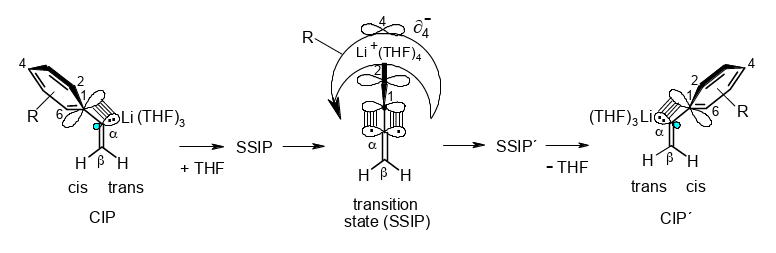
“Conducted Tour” Migration of Li+ during the cis/trans Stereoinversion of α-Arylvinyllithiums.
Rudolf Knorr, Claudia Behringer, Jakob Ruhdorfer, Ulrich von Roman, Ernst Lattke, and Petra Böhrer,
Chem. Eur. J. 2017, 23, 12861 – 12869.
-
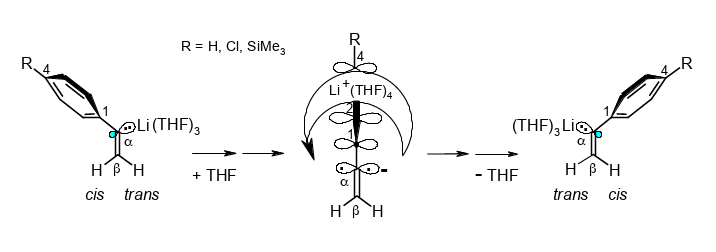
Why is cis/trans stereoinversion with Li+(THF)4 migration across the phenyl ring of α-lithiostyrene accelerated by two ortho-methyl groups?
Rudolf Knorr, Ernst Lattke, Jakob Ruhdorfer, Kathrin Ferchland, and Ulrich von Roman,
Tetrahedrom 2018, 74, 1621–1631.
-
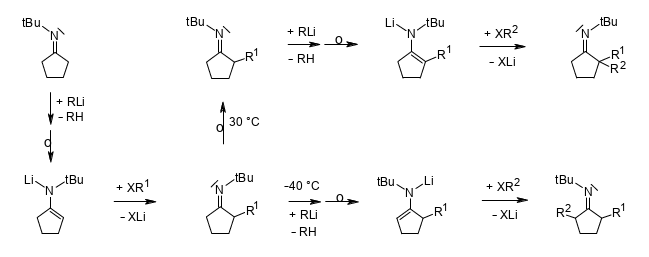
Regioselective Dialkylations of N-(tert-Butyl)iminocyclopentane via Deprotonating One-Pot Procedures.
Rudolf Knorr and Brigitte Neuner, Helv. Chim. Acta 2018, 101, e1800037,
DOI: 10.1002/hlca.201800037.
-
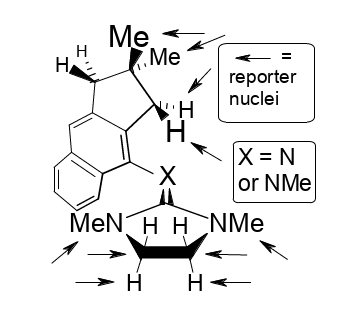
Demonstration of NC(sp2)-centered stereofluctuations in a guanidine derivative and its cation.
Rudolf Knorr and Petra Böhrer, J. Phys. Org. Chem. 2018, 31, e3833,
DOI: 10.1002/poc.3833.
-
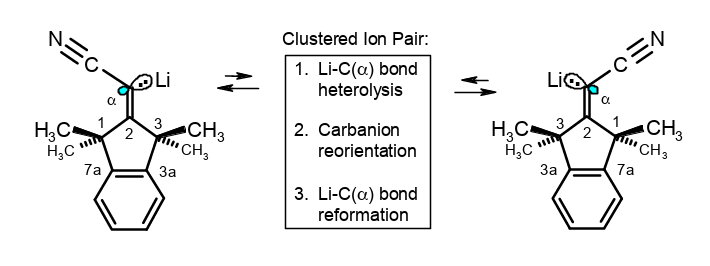
Structures and stereoinversions of β-shielded α-Li (or K or Cs)- acrylonitriles: A mechanism with neighborly assistance.
Rudolf Knorr, Barbara Schmidt, Johann Mehlstäubl, Therese von Roman, J. Organomet. Chem. 2018, 871, 185–196.
-
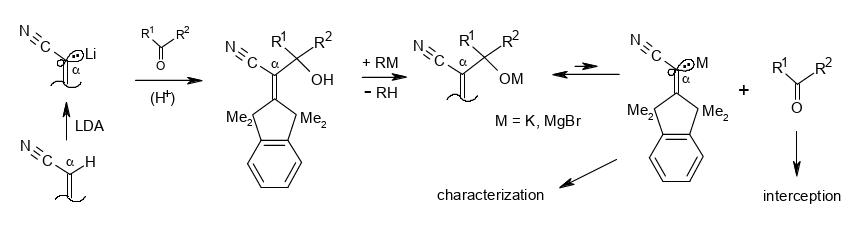
Nucleofugal behavior of a β-shielded α-cyanovinyl carbanion.
Rudolf Knorr and Barbara Schmidt, Beilstein J. Org. Chem. 2018, 14, 3018–3024,
DOI: 10.3762/bjoc.14.281
-
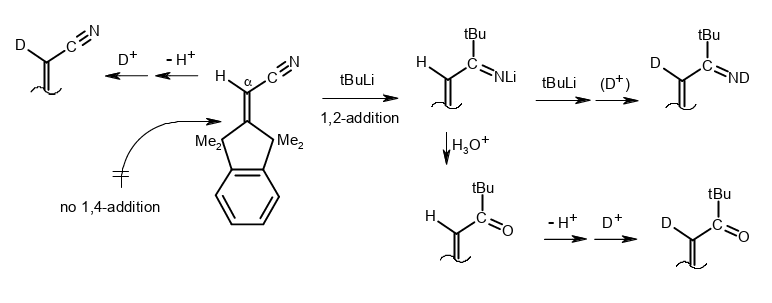
β-Shielded Michael systems whose H–C(sp2) deprotonation and 1,2-addition reactions compete for organolithiums.
Rudolf Knorr, Barbara Schmidt, Johannes Freudenreich, and Therese von Roman, Tetrahedron 2018, 74, 7466–7471.
-
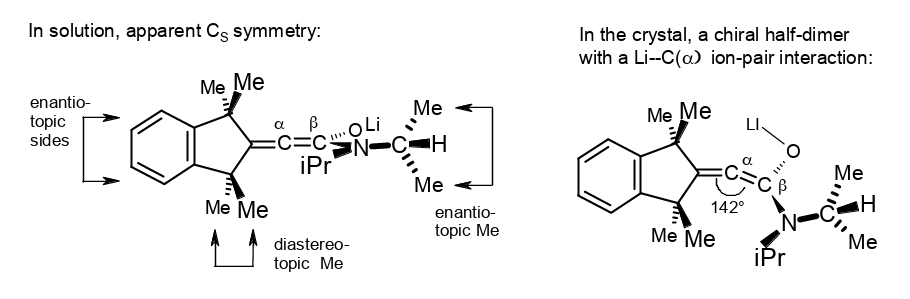
α-Deprotonation of a β-shielded acrylamide creates a distorted lithium 1-aminoallen-1-olate.
Rudolf Knorr, Barbara Schmidt, and Therese von Roman, J. Organomet. Chem. 2019, 894, 78–83.
-
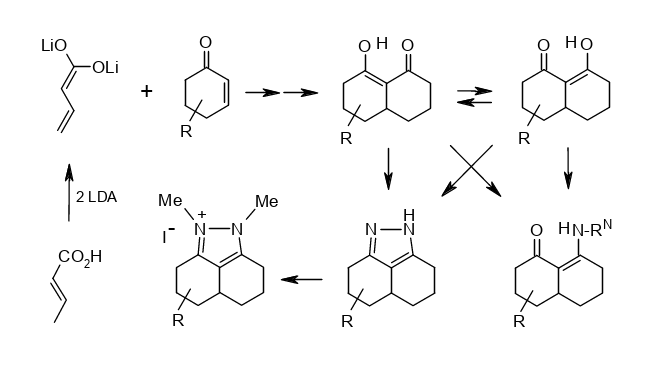
Short Syntheses of some “Decalin-1,8-diones” and their Derivatives: Breaking the Pretended Symmetry.
Rudolf Knorr, Annette Nadolny, Hermann Hauer, and Petra Böhrer, Helv. Chim. Acta 2019, 102, issue 6,e1800231, DOI: 10.1002/hlca.201800231.