Enamines, Enamides, and Ynamides
For reactivities of structurally related compounds go to: Nucleophiles » C-Nucleophiles
Found 74 Molecules.
| Molecule | Solvent | Reactivity Parameters | Classification | Reference (sort by title or year) |
|---|---|---|---|---|
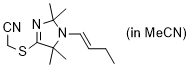 (E)-2-((1-(but-1-en-1-yl)-2,2,5,5-tetramethyl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 9.23 sN Parameter: 0.74 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
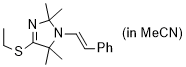 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 8.12 sN Parameter: 0.88 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
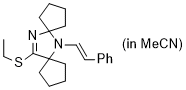 (E)-13-(ethylthio)-6-styryl-6,12-diazadispiro[4.1.47.25]tridec-12-ene (in MeCN) | MeCN | N Parameter: 7.79 sN Parameter: 0.87 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
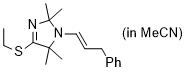 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-(3-phenylprop-1-en-1-yl)-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 9.64 sN Parameter: 0.70 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
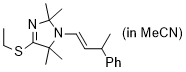 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-(3-phenylbut-1-en-1-yl)-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 7.92 sN Parameter: 0.73 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
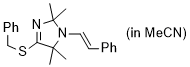 (E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in MeCN) | MeCN | N Parameter: 8.30 sN Parameter: 0.84 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
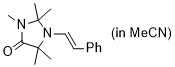 (E)-2,2,3,5,5-pentamethyl-1-styrylimidazolidin-4-one (in MeCN) | MeCN | N Parameter: 7.10 sN Parameter: 0.82 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
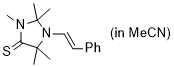 (E)-2,2,3,5,5-pentamethyl-1-styrylimidazolidine-4-thione (in MeCN) | MeCN | N Parameter: 6.49 sN Parameter: 0.83 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
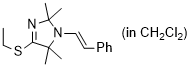 (E)-4-(ethylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 8.13 sN Parameter: 0.97 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
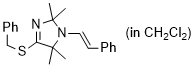 (E)-4-(benzylthio)-2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazole (in CH2Cl2) | dichloromethane | N Parameter: 7.99 sN Parameter: 0.98 | Chem. Eur. J. 2024, 30, e202302764 DOI: 10.1002/chem.202302764 | |
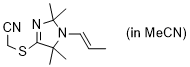 2-((2,2,5,5-tetramethyl-1-(prop-1-en-1-yl)-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 9.25 sN Parameter: 0.84 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
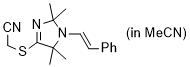 2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in MeCN) | MeCN | N Parameter: 7.74 sN Parameter: 0.87 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
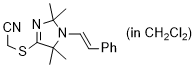 2-((2,2,5,5-tetramethyl-1-styryl-2,5-dihydro-1H-imidazol-4-yl)thio)acetonitrile (in CH2Cl2) | dichloromethane | N Parameter: 7.06 sN Parameter: 1.11 | Chem. Commun. 2023, 59, 8091-8084 DOI: 10.1039/d3cc01912h | |
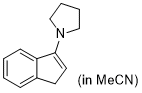 3-pyrrolidino-1H-indene (in MeCN) | MeCN | N Parameter: 15.27 sN Parameter: 0.93 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
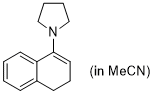 1-pyrrolidino-3,4-dihydronaphthalene (in MeCN) | MeCN | N Parameter: 14.09 sN Parameter: 0.66 | Synthesis 2019, 51, 1157-1170 DOI: 10.1055/s-0037-1611634 | |
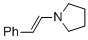 (E)-1-styrylpyrrolidine (in MeCN) | MeCN | N Parameter: 13.87 sN Parameter: 0.76 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
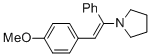 (E)-1-(2-(4-methoxyphenyl)-1-phenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 11.99 sN Parameter: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
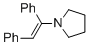 (E)-1-(1,2-diphenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 11.66 sN Parameter: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
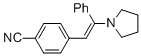 (E)-4-(2-phenyl-2-(pyrrolidin-1-yl)vinyl)benzonitrile (in MeCN) | MeCN | N Parameter: 10.63 sN Parameter: 0.84 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
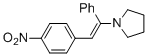 (E)-1-(2-(4-nitrophenyl)-1-phenylvinyl)pyrrolidine (in MeCN) | MeCN | N Parameter: 10.42 sN Parameter: 0.82 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
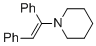 (E)-1-(1,2-diphenylvinyl)piperidine (in MeCN) | MeCN | N Parameter: 9.94 sN Parameter: 0.86 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
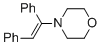 (E)-4-(1,2-diphenylvinyl)morpholine (in MeCN) | MeCN | N Parameter: 8.78 sN Parameter: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
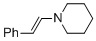 (E)-beta-(N-piperidino)styrene (in MeCN) | MeCN | N Parameter: 13.84 sN Parameter: 0.73 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
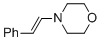 (E)-4-styrylmorpholine | MeCN | N Parameter: 11.66 sN Parameter: 0.83 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
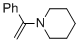 alpha-(N-piperidino)styrene (in MeCN) | MeCN | N Parameter: 11.60 sN Parameter: 0.80 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
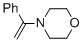 alpha-(N-morpholino)styrene (in MeCN) | MeCN | N Parameter: 10.30 sN Parameter: 0.80 | Chem. Eur. J. 2018, 24, 5901-5910 DOI: 10.1002/chem.201705962 | |
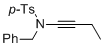 N-benzyl-N-(but-1-yn-1-yl)-4-methylbenzenesulfonamide | dichloromethane | N Parameter: 5.16 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
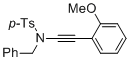 N-benzyl-N-((2-methoxyphenyl)ethynyl)-4-methylbenzenesulfonamide | dichloromethane | N Parameter: 4.40 sN Parameter: 0.86 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
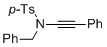 4-methyl-N-(2-phenylethynyl)-N-(phenylmethyl)benzenesulfonamide | dichloromethane | N Parameter: 3.85 sN Parameter: 0.84 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
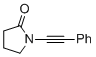 1-(phenylethynyl)pyrrolidin-2-one | dichloromethane | N Parameter: 3.12 sN Parameter: 0.85 | Angew. Chem. Int. Ed. 2014, 53, 4968-4971 DOI: 10.1002/anie.201402055 | |
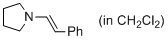 (E)-1-styrylpyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 12.26 sN Parameter: 0.93 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
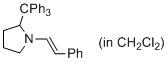 (E)-1-styryl-2-tritylpyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 10.19 sN Parameter: 1.06 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
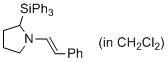 (E)-1-styryl-2-(triphenylsilyl)pyrrolidine (in CH2Cl2) | dichloromethane | N Parameter: 11.77 sN Parameter: 0.98 | J. Am. Chem. Soc. 2014, 136, 14263-14269 DOI: 10.1021/ja508065e | |
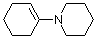 1-(N-piperidino)cyclohexene (in MeCN) | MeCN | N Parameter: 14.02 sN Parameter: 0.76 | J. Am. Chem. Soc. 2013, 135, 6579-6587 DOI: 10.1021/ja401106x | |
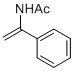 N-(1-phenylvinyl)acetamide | MeCN | N Parameter: 5.73 sN Parameter: 0.97 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
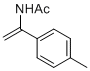 N-(1-(p-tolyl)vinyl)acetamide | MeCN | N Parameter: 6.57 sN Parameter: 0.91 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
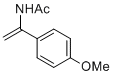 N-(1-(4-methoxyphenyl)vinyl)acetamide | MeCN | N Parameter: 7.06 sN Parameter: 0.85 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
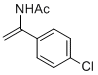 N-(1-(4-chlorophenyl)vinyl)acetamide | MeCN | N Parameter: 5.60 sN Parameter: 1.00 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
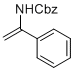 benzyl (1-phenylvinyl)carbamate | MeCN | N Parameter: 6.21 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
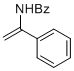 N-(1-phenylvinyl)benzamide | MeCN | N Parameter: 5.44 sN Parameter: 1.00 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
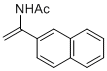 N-(1-(naphthalen-2-yl)vinyl)acetamide | MeCN | N Parameter: 6.28 sN Parameter: 0.95 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
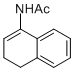 N-(3,4-dihydronaphthalen-1-yl)acetamide | MeCN | N Parameter: 4.91 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
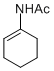 N-(cyclohex-1-en-1-yl)acetamide | MeCN | N Parameter: 5.64 sN Parameter: 0.79 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
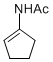 N-(cyclopent-1-en-1-yl)acetamide | MeCN | N Parameter: 7.06 sN Parameter: 0.87 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
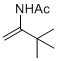 N-(3,3-dimethylbut-1-en-2-yl)acetamide | MeCN | N Parameter: 4.61 sN Parameter: 0.98 | Chem. Eur. J. 2012, 18, 5732-5740 DOI: 10.1002/chem.201103519 | |
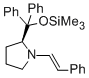 (S,E)-2-(diphenyl(trimethylsiloxy)methyl)-1-styrylpyrrolidine | MeCN | N Parameter: 10.56 sN Parameter: 1.01 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
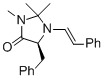 (S,E)-5-benzyl-2,2,3-trimethyl-1-styrylimidazolidin-4-one | MeCN | N Parameter: 7.20 sN Parameter: 1.14 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
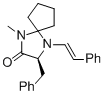 (S,E)-3-benzyl-1-methyl-4-styryl-1,4-diazaspiro[4.4]nonan-2-one | MeCN | N Parameter: 7.92 sN Parameter: 1.07 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
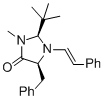 (2S,5S)-5-benzyl-2-(tert-butyl)-3-methyl-1-((E)-styryl)imidazolidin-4-one | MeCN | N Parameter: 5.80 sN Parameter: 0.87 | Angew. Chem. Int. Ed. 2012, 51, 5739-5742 DOI: 10.1002/anie.201201240 | |
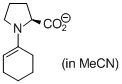 (cyclohexen-1-yl)prolinate (in MeCN) | MeCN | N Parameter: 18.86 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
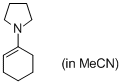 1-(N-pyrrolidino)cyclohexene (in MeCN) | MeCN | N Parameter: 16.42 sN Parameter: 0.70 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
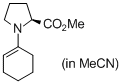 methyl (cyclohexen-1-yl)prolinate (in MeCN) | MeCN | N Parameter: 14.96 sN Parameter: 0.68 | Angew. Chem. Int. Ed. 2010, 49, 9526-9529 DOI: 10.1002/anie.201004344 | |
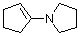 1-(N-pyrrolidino)cyclopentene | dichloromethane | N Parameter: 15.91 sN Parameter: 0.86 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
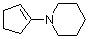 1-(N-piperidino)cyclopentene | dichloromethane | N Parameter: 15.06 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
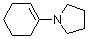 1-(N-pyrrolidino)cyclohexene | dichloromethane | N Parameter: 14.91 sN Parameter: 0.86 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
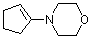 1-(N-morpholino)cyclopentene | dichloromethane | N Parameter: 13.41 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
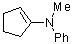 1-(phenylmethylamino)cyclopentene | dichloromethane | N Parameter: 12.90 sN Parameter: 0.79 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
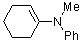 1-(phenylmethylamino)cyclohexene | dichloromethane | N Parameter: 10.73 sN Parameter: 0.81 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
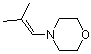 morpholinoisobutylene | dichloromethane | N Parameter: 10.04 sN Parameter: 0.82 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
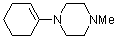 1-(1-cyclohexenyl)-4-methylpiperazine | dichloromethane | N Parameter: 12.51 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
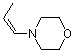 (Z)-1-(N-morpholino)propene | dichloromethane | N Parameter: 12.26 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
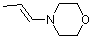 (E)-1-(N-morpholino)propene | dichloromethane | N Parameter: 12.06 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
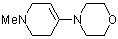 1-methyl-4-(N-morpholino)tetrahydropyridine | dichloromethane | N Parameter: 12.03 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
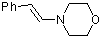 (E)-beta-(N-morpholino)styrene | dichloromethane | N Parameter: 10.76 sN Parameter: 0.87 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
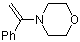 alpha-(N-morpholino)styrene | dichloromethane | N Parameter: 9.96 sN Parameter: 0.79 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
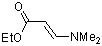 ethyl (E)-3-(dimethylamino)acrylate | dichloromethane | N Parameter: 9.43 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
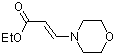 ethyl (E)-3-(N-morpholino)acrylate | dichloromethane | N Parameter: 8.52 sN Parameter: 0.80 | Chem. Eur. J. 2003, 9, 2209-2218 DOI: 10.1002/chem.200204666 | |
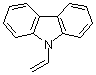 N-vinylcarbazole | dichloromethane | N Parameter: 5.02 sN Parameter: 0.94 | Macromolecules 2002, 35, 5454-5458 DOI: 10.1021/ma020306l | |
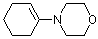 1-(N-morpholino)cyclohexene | dichloromethane | N Parameter: 11.40 sN Parameter: 0.83 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
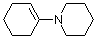 1-(N-piperidino)cyclohexene | dichloromethane | N Parameter: 13.36 sN Parameter: 0.81 | J. Am. Chem. Soc. 2001, 123, 9500-9512 DOI: 10.1021/ja010890y | |
 2-(bis(trimethylsiloxy)amino)propene | dichloromethane | N Parameter: 4.76 sN Parameter: 0.86 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
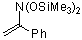 (bis(trimethylsiloxy)amino)styrene | dichloromethane | N Parameter: 4.80 sN Parameter: 0.86 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
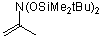 2-(bis(tert-butyldimethylsiloxy)amino)propene | dichloromethane | N Parameter: 4.23 sN Parameter: 0.93 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 | |
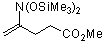 4-(bis(trimethylsiloxy)amino)pent-4-enoic acid methyl ester | dichloromethane | N Parameter: 3.84 sN Parameter: 0.87 | J. Org. Chem. 2001, 66, 3196-3200 DOI: 10.1021/jo0015927 |