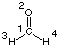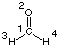Natural Population Analysis
Link 607 of Gaussian contains version 3.1 of the NBO program by F. Weinhold and coworkers.
Using formaldehyde (CH2O, C2v symmetry) at the HF/STO-3G level as an example,
the calculation of NPA charges only involves calculation of the natural atomic orbitals and
summation over all NAOs of a given atom to obtain the Natural Charges for each of the atoms.
The corresponding input file is:
#P HF/STO-3G scf=tight pop=npa
HF/STO-3G//HF/STO-3G sp formaldehyde
0 1
C1
O2 1 r2
H3 1 r3 2 a3
H4 1 r3 2 a3 3 180.0
r2=1.21672286
r3=1.10137241
a3=122.73666566
| |
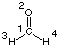 |
The following output is obtaind using this input:
******************************Gaussian NBO Version 3.1******************************
N A T U R A L A T O M I C O R B I T A L A N D
N A T U R A L B O N D O R B I T A L A N A L Y S I S
******************************Gaussian NBO Version 3.1******************************
/RESON / : Allow strongly delocalized NBO set
Analyzing the SCF density
Job title: test21f HF/STO-3G opt formaldehyde scf=conventional
Storage needed: 510 in NPA ( 2000000 available)
NATURAL POPULATIONS: Natural atomic orbital occupancies
NAO Atom No lang Type(AO) Occupancy Energy
---------------------------------------------------------
1 C 1 s Cor( 1s) 2.00000 -11.05947
2 C 1 s Val( 2s) 1.08461 -0.26145
3 C 1 px Val( 2p) 0.90165 -0.04494
4 C 1 py Val( 2p) 0.99304 0.07032
5 C 1 pz Val( 2p) 0.85379 0.14739
6 O 2 s Cor( 1s) 1.99999 -20.15602
7 O 2 s Val( 2s) 1.79982 -1.09031
8 O 2 px Val( 2p) 1.09835 -0.11626
9 O 2 py Val( 2p) 1.91857 -0.38126
10 O 2 pz Val( 2p) 1.37055 -0.15404
11 H 3 s Val( 1s) 0.98982 0.02250
12 H 4 s Val( 1s) 0.98982 0.02250
Summary of Natural Population Analysis:
Natural Population
Natural -----------------------------------------------
Atom No Charge Core Valence Rydberg Total
-----------------------------------------------------------------------
C 1 0.16692 2.00000 3.83308 0.00000 5.83308
O 2 -0.18728 1.99999 6.18729 0.00000 8.18728
H 3 0.01018 0.00000 0.98982 0.00000 0.98982
H 4 0.01018 0.00000 0.98982 0.00000 0.98982
=======================================================================
* Total * 0.00000 3.99999 12.00001 0.00000 16.00000
Natural Population
--------------------------------------------------------
Core 3.99999 ( 99.9997% of 4)
Valence 12.00001 (100.0001% of 12)
Natural Minimal Basis 16.00000 (100.0000% of 16)
Natural Rydberg Basis 0.00000 ( 0.0000% of 16)
--------------------------------------------------------
Atom No Natural Electron Configuration
----------------------------------------------------------------------------
C 1 [core]2s( 1.08)2p( 2.75)
O 2 [core]2s( 1.80)2p( 4.39)
H 3 1s( 0.99)
H 4 1s( 0.99)
A full online-manual for the NBO program explaining some of the underlying concepts
and all additional keywords can be found
here.
The Weinhold group also offers updated versions of the NBO program. The corresponding
web page
also contains substantial additional information on the concept of NBO and NPA.
A detailed overview of the performance of the NPA method in describing the charge distribution
in water can be found in:
F. Martin, H. Zipse, J. Comp. Chem. 2005, 26, 97 - 105.
A copy of the pdf file is available here.
last changes: 09.01.2005, HZ
questions & comments to: zipse@cup.uni-muenchen.de