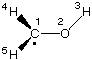
#SVWN/6-31G(d) freq SVWN/6-31G(d) freq methanol-1-yl radical 0 2 C1 O2 1 r2 H3 2 r3 1 a3 H4 1 r4 2 a4 3 d4 H5 1 r5 2 a5 4 d5 r2=1.35454381 r3=0.97675734 r4=1.09713107 r5=1.09208721 a3=108.95266528 a4=119.30335589 a5=113.0329959 d4=-26.58949552 d5=-149.77421707